Ionomers
Understanding and Optimizing Polymeric Materials
Our research involves characterization and modeling of the transport and mechanical properties of various ion-conductive polymers, composites and thin films, and their structural characterization to establish a structure/function relationship for improving their performance and durability in energy conversion devices. Our research focuses on ion-conducting polymers, especially their existence as both membranes (i.e., solid-electrolytes) and electrolyte thin films (in electrode structures) for various electrochemical technologies. Any factor that improves transport properties of an ionomer tend to undermine its stability, thereby creating an intriguing transport/stability trade-off. An active area of research is to understand how ionomer chemistry and environmental factors control the interplay between the transport and stability of the ionomers and to develop high-performance and durable materials for electrochemical energy devices.
Areas of Focus
- Ionomers for Polymer-Electrolyte Fuel Cells (PEFCs) in both membrane and thin films forms for improved performance and durability
- Ionomers for Electrolyzers, water-splitting device and applications for improved separator performance and efficiency
- Ionomers for Redox Flow Batteries (RFBs) for optimized transport and selectivity of species
- Advanced diagnostics of ion-exchange membranes for transport and mechanical properties under controlled environment
- Multi-scale modeling of ionomers for various applications
- Development of characterization techniques for morphology and mechanical properties of ionomer membranes and thin films
- Novel synchrotron X-ray characterization techniques for probing structure of ionomer membranes and composite separators
Structure-Function Characterization for Fuel Cells
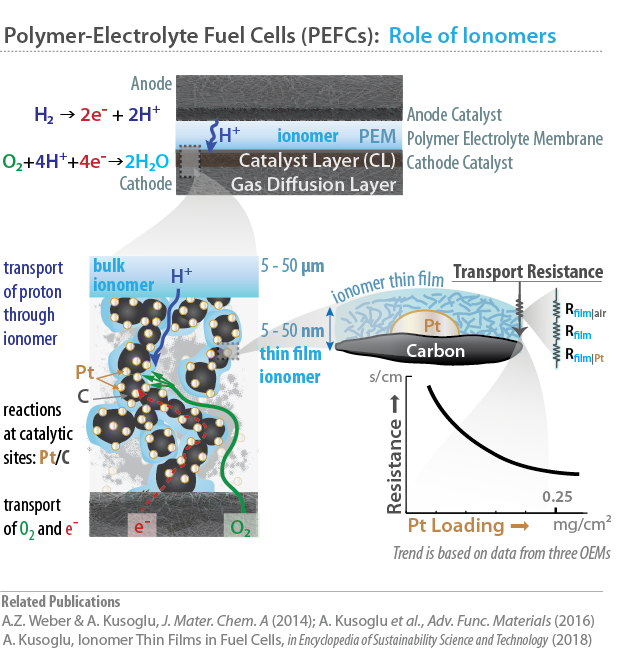
Ionomers play a key role in ion transport in a PEFC, as the polymer-electrolyte membrane (PEM) that selectively transports protons from anode to cathode, and as the electrolyte film within the catalyst layers (CLs), wherein electrochemical reactions occur. In a CL, these ionomers are found as nanometer-thick electrolyte “thin film” binding the catalytic sites (C, Pt) where the charges, ions and species meet to react. In such heterogeneous structures, substrate/film interactions affect the transport limitations and therefore the performance. The latter phenomenon could have important implications on explaining the mass-transport limitations in CLs, which are thought to be related to the transport resistances at the ionomer thin-film.
Performance and durability of the ionomers in PEFCs are related to their properties governed by multi-scale morphology and interactions between chemical structure and water, all of which change with the environment. Moreover, the ionomer behaves differently from the bulk (PEM) when it is confined to nanometer thicknesses (as in electrodes), where its behavior is strongly influenced by its interfaces with the air and the particles. Thus, it is of interest to understand and optimize transport functionalities and mechanical stability across the lengthscales, i.e. from bulk to thin film.
Our current research is on the structure/function relationship of thin films of various ionomer chemistries, including, but not limited to, perfluorosulfonic acid (PFSA) ionomers. We examine the ionomer thin-film morphology from micrometer to nanometer lengthscales, in-situ using advanced synchotron X-ray techniques (at the Advanced Light Source), and employ related advanced characterization techniques, to enable correlations between an ionomer’s phase-separated nanostructure and its transport and mechanical properties.
Separators for Electrolyzers and Flow Batteries
A common use of solid-electrolyte membrane in energy technologies is proton-conduction, in which case the ionomer functions as a proton-exchange membrane (PEM).
Links
- Advanced Light Source website: als.lbl.gov
