Ceramics
Stronger Components for High-Temperature Fuel Cells
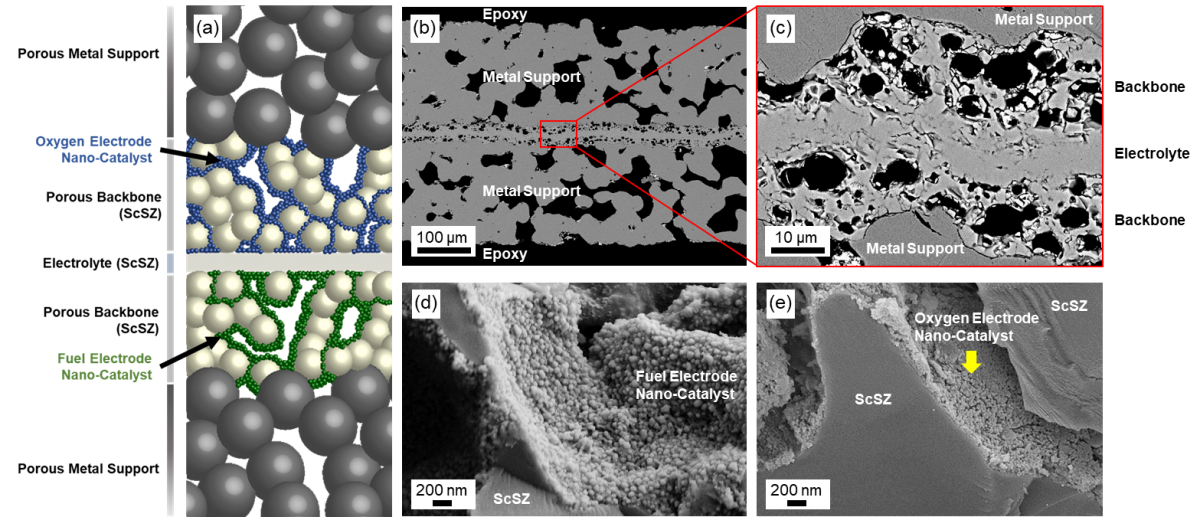
In solid-oxide fuel cells, all of the electrochemistry takes place within a thin, ceramic electrolyte layer that converts fuel and oxygen to electricity. We are working on mounting ceramic layers on porous stainless steel supports, which are lower in cost and more mechanically rugged than all-ceramic fuel cells, which can shatter when temperatures change rapidly. Our focus is on understanding and overcoming the processing limitations of fabricating ceramics on these porous metal substrates.
This work is addressing the need for fuel cells that can operate within fast-start, dynamic temperature environments that would shatter conventional all-ceramic versions, from personal power products than can immediately charge a cell phone to range extenders for electric cars. We are also developing electrolysis cells based on the same architecture for production of hydrogen and conversion of carbon dioxide using rapidly variable power input such as solar panels or wind turbines.