Fuel Cells
Building a Future Power Source
Our group is working to understand and optimize the next generation fuel-cell and related energy-conversion and energy-storage components and materials, mainly through physics-based multi-scale modeling of cell behavior, advanced diagnostics of cell properties, and synthesis of novel key materials. Our core team includes electrochemists, chemical engineers, mechanical engineers, theorists, material scientists and organic chemists. We also collaborate extensively with labs, industry and academia.
Current research interests related to fuel cells include:
- Mathematical modeling of the underlying physics at the cell and component level for polymer-electrolyte fuel cells (PEFCs) at low temperatures
- Fuel-cell component degradation studies to increase cell durability
- Characterization of fuel-cell components including ionomer membranes, catalyst layers, and diffusion media
- Modeling and diagnostics studies for Fuel-Cell Performance and Durability (FC-PAD) consortium
- Examination of structure/function/property relationships of ion-conducting polymers and thin films
- Development of next-generation separators for alkaline and PEM fuel cells
- Manufacturing metrology diagnostic development for fuel cells
Low-Temperature Fuel Cells: PEFCs
Polymer-electrolyte fuel-cells (PEFCs) are at the forefront of electrochemical energy-conversion technologies with their great potential to offer clean and renewable sustainable-energy technologies for stationary and transportation applications. To commercialize successfully PEFCs, the cost must be reduced without sacrificing performance and lifetime. A polymer electrolyte fuel cell (PEFC) is comprised of multiple functional layers and combinations of materials and involve with varying physical processes such as ion conduction, multiphase fluid flow, chemical reactions, and generally require the transport of charged intermediates (e.g, protons, hydroxide anions, redox cations) through a solid-state ion-conducting polymer membrane or ionomer and into inorganic catalytic inclusions where redox reactions occur. The oxidation of a H2-rich fuel at the anode generates electrons and protons; and while electrons move through an external circuit to generate electricity, protons pass through the electrolyte to reach cathode, where they meet electrons and reduce O2 to water (Figure). Due to the formation of water, the 4-electron process of O2 reduction, and the use of air as the O2 carrier, the overall reaction rate is sluggish and represents the largest inefficiency of cell operation, which is currently considered one of the most critical challenges that must be addressed (see a perspective on the topic for further reading). Similarly, there exists a challenge to simultaneously improve performance and durability in ionomer membranes (see our perspective on the topic for further reading).
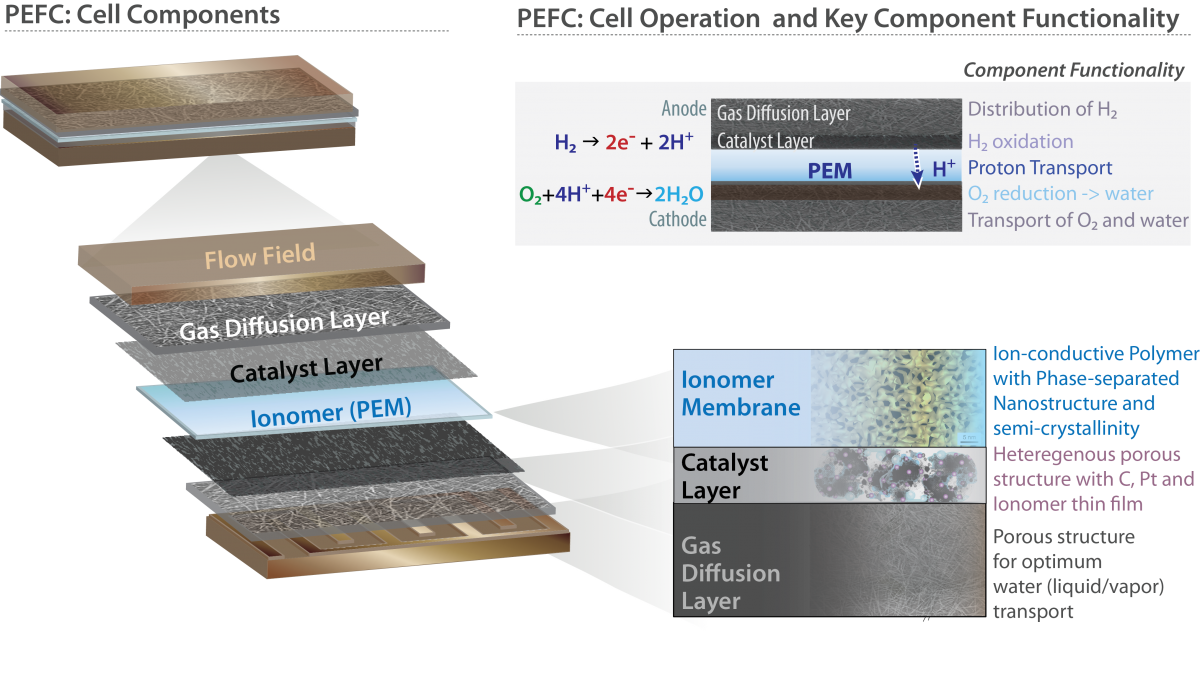
Our research activities are centered around structure-property characterization and performance diagnostics of these components:
- Gas diffusion layer (GDL), a porous structure facilitating distribution of species
- Catalyst layers (CLs), complex heterogeneous porous structures wherein the electrochemical reactions occur
- Polymer-electrolye membrane (PEM), an ion-conductive polymer (ionomer) typically with additives for improved performance and durability
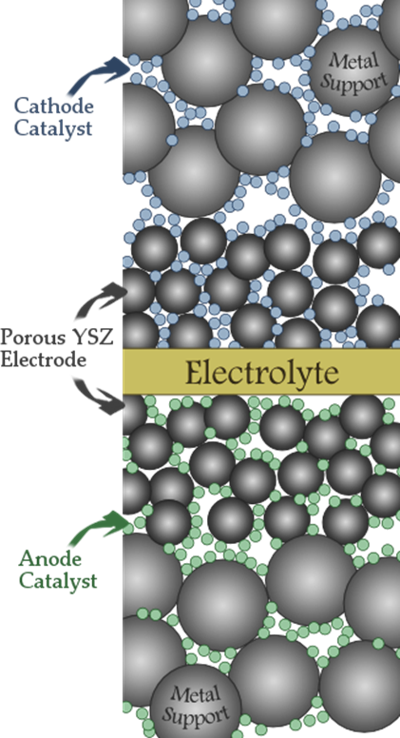
High-Temperature Fuel Cells: SOFCs
Metal-supported Solid Oxide Fuel Cells (SOFC) are rugged, cost-effective cells that are well suited for portable and mobile applications requiring abuse tolerance, rapid start-up capability, and fuel flexibility. LBNL has been a leader in this field since the early 2000’s and now conducts projects aiming to produce direct-propane-powered personal power product prototypes, and ethanol-fueled traction-power cell technology for SOFC-Battery hybrid passenger vehicles.
Links
- "Unexplained Transport Resistances for Low-Loaded Fuel-Cell Catalyst Layers": energyconversiongroup.lbl.gov/publications/unexplained-transport-resistances-low
- "Electrochemical/Mechanical Coupling in Ion-Conducting Soft Matter": energyconversiongroup.lbl.gov/publications/electrochemicalmechanical-coupling
- Fuel-Cell Consortium for Performance and Durability website: www.fcpad.org

